In principle once started a radical polymerization might be expected to continue unchecked producing a few extremely long chain polymers.
Chain growth polymerization of vinyl chloride.
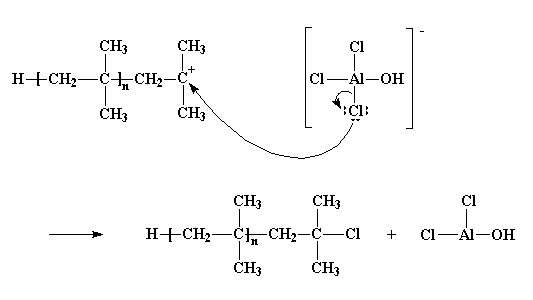
Polymerization of isobutylene 2 methylpropene by traces of strong acids is an example of cationic polymerization.
The following animation illustrates the polymerization of vinyl chloride initiated by radicals.
To make the polyvinyl chloride pvc as per the requirements of the consumer market many other additives are added into it.
Chain reaction polymerization sometimes called addition polymerization requires an initiator to start the growth of the reaction.
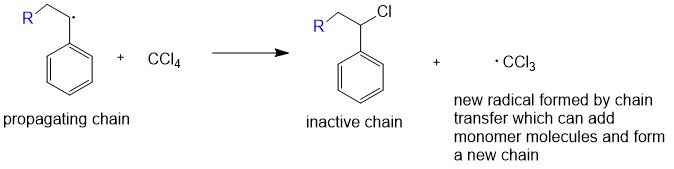
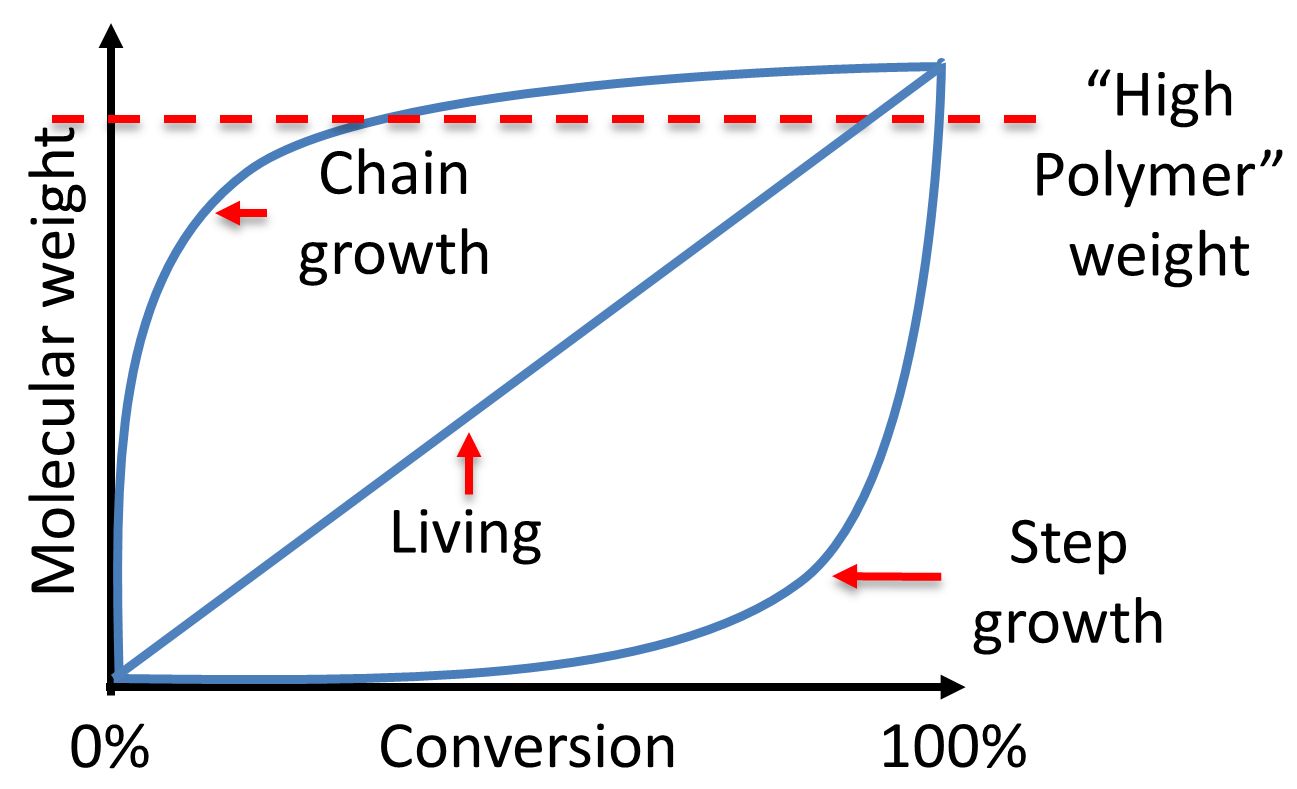
There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
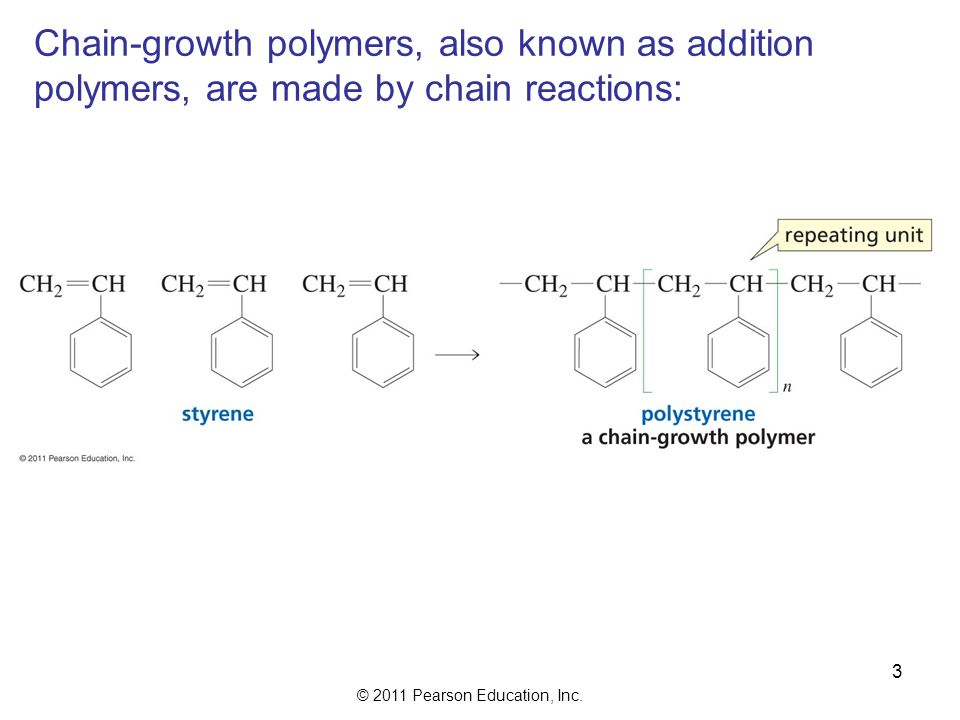
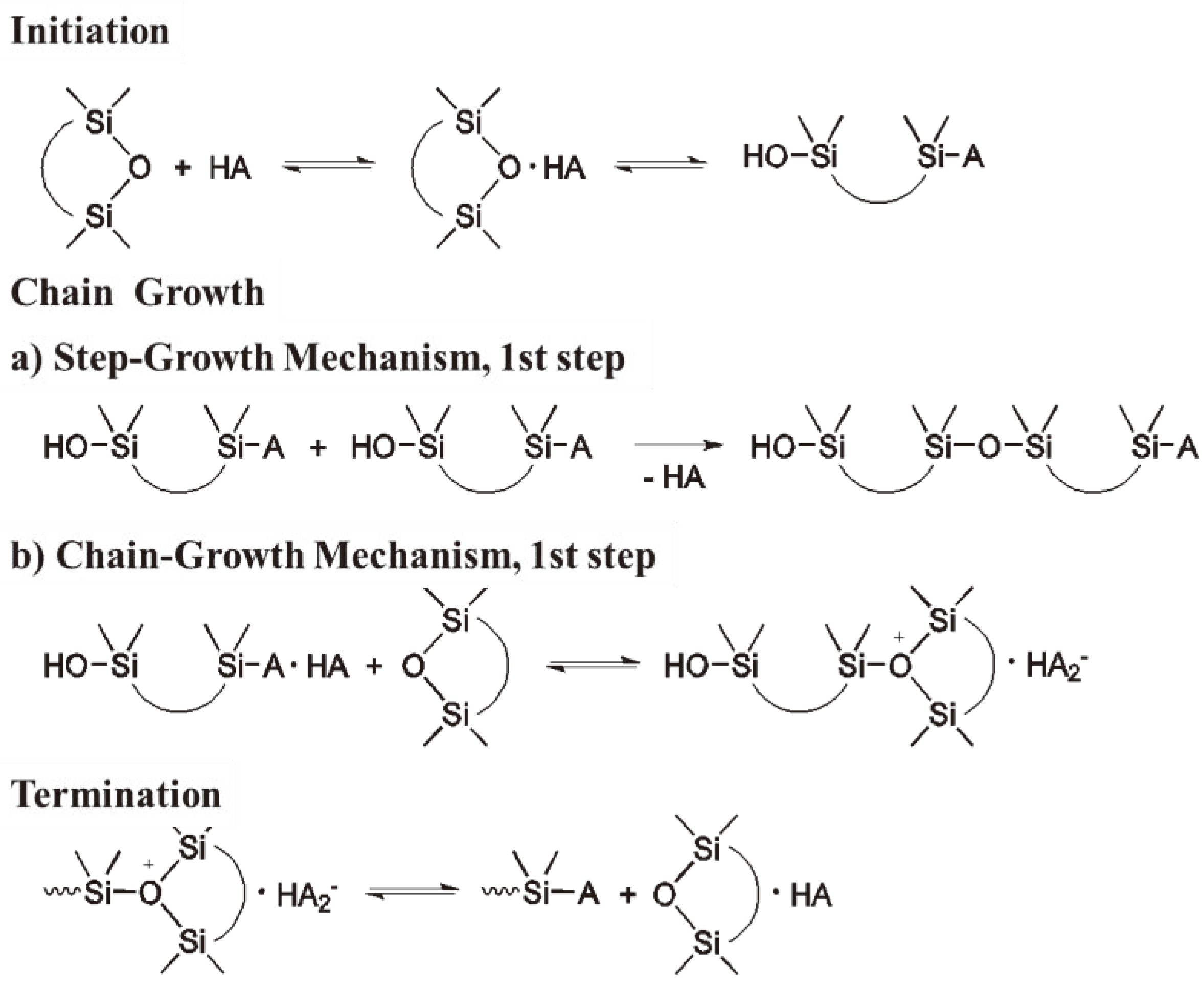
Chain growth polymerization american spelling or chain growth polymerisation english spelling is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time.
Radical chain growth polymerization of vinyl chloride.
The largest family of polymers 3 vinyl polymers are produced by chain polymerization reactions a good example is the free radical polymerization of styrene which is initiated by a free radical r that reacts with styrene.
The main problem is the amount of r vcm in the final pvc slurry which has to be less than 1.
With chain polymerization what are termed vinyl polymers are obtained because the monomers from which it starts contain the vinyl group.
Free radical polymerization frp is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks.
The polymerization of vinyl monomers occurs by rupture of the double bond and creation of a simple covalent bond with the nearby monomer.
In slurry polymerization chain growth occurs as monomers dissolved in a hydrocarbon solvent add to growing chains also dissolved in the solvent.
The polymerization reaction in the reactor takes place by means of chain growth polymerization of vinyl chloride monomer vcm.
The in species represents the initiating radical which might originate from a peroxide or azo initiator.
This process is similar to radical polymerization as demonstrated by the following equations.
Ch 2 ch in which the c c double bond is present.
Following its generation the initiating free radical adds nonradical monomer units thereby growing the polymer chain.
A typical polymerization includes 180 parts water and 100 parts vinyl chloride monomer chain transfer agent trichloro ethylene.
The polyisobutylene product is a soft rubbery solid tg 70º c which is used for inner tubes.
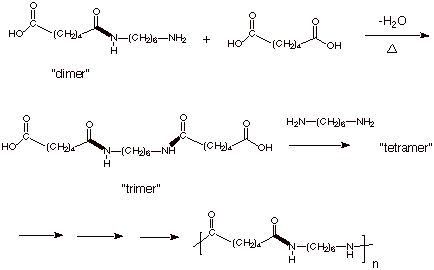
Chain polymerization of vinyl monomers is a convenient technique for the preparation of a wide variety of functional polymers.
The reactants are then heated in the closed system to about 50 c and the pressure rises to about 0 5 mpa.
To see an animated model of the radical chain growth polymerization of vinyl chloride.